CAPA and Root Cause Analysis MasterClass - US edition
14-15 April, 2026
Broader framework of QMS
Participants will gain a clear understanding of how to apply the principles of Root Cause Analysis effectively, translate findings into effective Corrective and Preventive Action plans, and embed these practices within the broader framework of Quality management system and Risk-Based Quality Management, as endorsed by ICH E6(R3).
14-15 April, 2026, Virtual
STARTING TIME EDT 08:00 A.M. | PDT 05:00 A.M. | UTC 12:00 P.M.
Overview
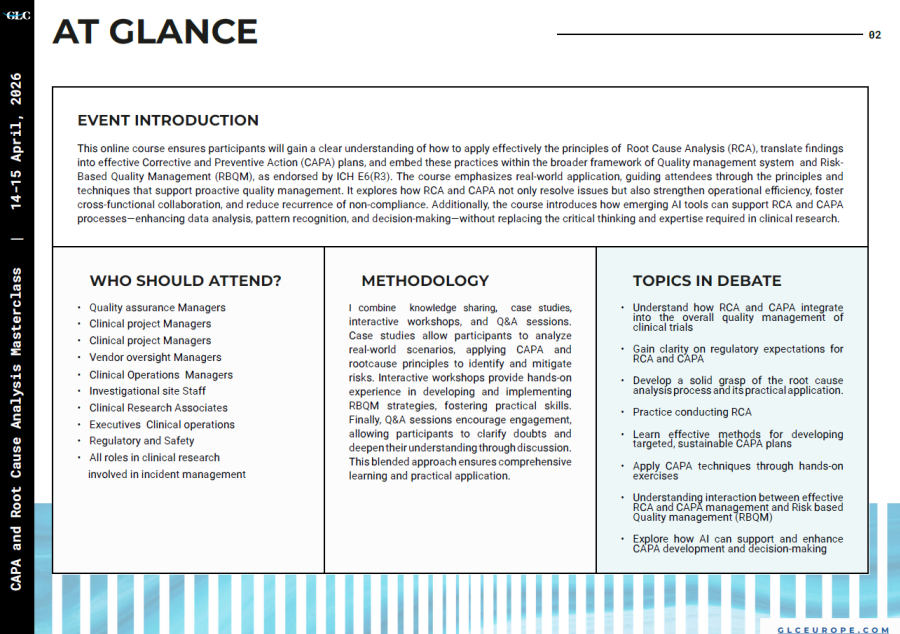
This online course ensures participants will gain a clear understanding of how to apply effectively the principles of Root Cause Analysis (RCA), translate findings into effective Corrective and Preventive Action (CAPA) plans, and embed these practices within the broader framework of Quality management system and Risk Based Quality Management (RBQM), as endorsed by ICH E6(R3). The course emphasizes real-world application, guiding attendees through the principles and techniques that support proactive quality management. It explores how RCA and CAPA not only resolve issues but also strengthen operational efficiency, foster cross-functional collaboration, and reduce recurrence of non-compliance.
Additionally, the course introduces how emerging AI tools can support RCA and CAPA processesenhancing data analysis, pattern recognition, and decision-makingwithout replacing the critical thinking and expertise required in clinical research.
#masterclass #glceurope #pharmaonlinetraining #globalleadingconferences #CAPA #rootcause #analysis #RBQM #RBQM
 WHO SHOULD ATTEND
WHO SHOULD ATTEND
-Quality assurance Managers
-Clinical project Managers
-Clinical project Managers
-Vendor oversight Managers
-Clinical Operations Managers
-Investigational site Staff
-Clinical Research Associates
-Executives Clinical operations
-Regulatory and Safety
-All roles in clinical research involved in incident management
Testimonial
Our success stories
"High attention to detail in course content and very well delivered"
Simon Halsey
Product Development Manager
Essentra Packaging
United Kingdom
Our success stories
"Very good training led by two knowledgeable and open experts. Excellent insight given on many complex topics. Interactive and highly useful"
Aurelie Vivicorsi
USP PD Team Manager
Celonic AG
Switzerland
Our success stories
"Great course, impressed with the knowledge of the trainers and ability to answer wide variety of questions!"
Emilia Szwej
Manager, Senior Investigator
MT Sword Laboratories (BMS)
Germany
About GLC
Global Leadership Conferences began as an ambitious dream by three founders ten years ago. Today its an international series of interactive events, exploring the hottest topics in critical fields. Each year, thousands of professionals join us to challenge the status quo and learn innovative ways to create new solutions in Finance, Pharmaceutical, HR, Health & Safety, and Energy.
know more >14+
Years of experience
600+
Events organized
4,000+
Speakers
25,000+
Attendees
Other events you may like

Training Program for CMC Leaders - EU edition
14th September 2026 - 09th April 2027
Rich with practical insights and real-world applications
learn more >>
Training Program for CMC Leaders - US edition
14th September 2026 - 09th April 2027
Rich with practical insights and real-world applications
learn more >>
Fundamentals of Toxicology MasterClass - EU edition
09-12 March, 2026
Applied Toxicology and its critical role in the therapeutic development lifecycle
learn more >>


























