Fundamentals of Toxicology MasterClass - US edition
09-12 March, 2026
Toxicology and GLP
This will allow non-toxicologists to learn the jargon and be able to effectively communicate with colleagues. In addition, the MasterClass will describe the basics of toxicology to allow non-specialists to understand the content of a toxicology report.
09-12 March, 2026, Virtual
Time Zone: Eastern Standard Time (EST) / UTC05:00
Overview
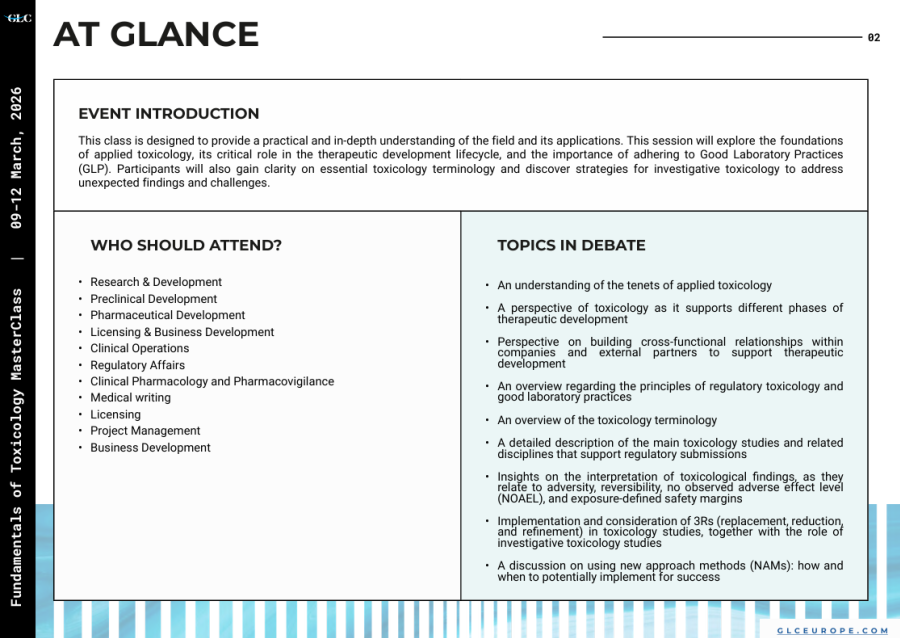
This class is designed to provide a practical and in-depth understanding of the field and its applications. This session will explore the foundations of applied toxicology, its critical role in the therapeutic development lifecycle, and the importance of adhering to Good Laboratory Practices (GLP). Participants will also gain clarity on essential toxicology terminology and discover strategies for investigative toxicology to address unexpected findings and challenges.
#masterclass #glceurope #pharmaonlinetraining #globalleadingconferences #Toxicology #regulatory
 WHO SHOULD ATTEND
WHO SHOULD ATTEND
-Research & Development
-Preclinical Development
-Pharmaceutical Development
-Licensing & Business Development
-Clinical Operations
-Regulatory Affairs
-Clinical Pharmacology and Pharmacovigilance
-Medical writing
-Licensing
-Project Management
-Business Development
Testimonial
Our success stories
"High attention to detail in course content and very well delivered"
Simon Halsey
Product Development Manager
Essentra Packaging
United Kingdom
Our success stories
"Very good training led by two knowledgeable and open experts. Excellent insight given on many complex topics. Interactive and highly useful"
Aurelie Vivicorsi
USP PD Team Manager
Celonic AG
Switzerland
Our success stories
"Great course, impressed with the knowledge of the trainers and ability to answer wide variety of questions!"
Emilia Szwej
Manager, Senior Investigator
MT Sword Laboratories (BMS)
Germany
About GLC
Global Leadership Conferences began as an ambitious dream by three founders ten years ago. Today its an international series of interactive events, exploring the hottest topics in critical fields. Each year, thousands of professionals join us to challenge the status quo and learn innovative ways to create new solutions in Finance, Pharmaceutical, HR, Health & Safety, and Energy.
know more >14+
Years of experience
600+
Events organized
4,000+
Speakers
25,000+
Attendees
Other events you may like

Training Program for CMC Leaders - EU edition
14th September 2026 - 09th April 2027
Rich with practical insights and real-world applications
learn more >>
Training Program for CMC Leaders - US edition
14th September 2026 - 09th April 2027
Rich with practical insights and real-world applications
learn more >>
Fundamentals of Toxicology MasterClass - EU edition
09-12 March, 2026
Applied Toxicology and its critical role in the therapeutic development lifecycle
learn more >>


























