Free knowledge to monitor the world of events. Have a look at our must read Blogs on Pharma, Finance, HR, Health and Cross Industry.
Development of LC/MS for Regulated Bioanalysis
2021-11-17
The rapid development of Liquid Chromatography/Mass Spectrometry (LC/MS) technology changed the way bioanalysis was done. Regulated Bioanalysis services are valuable to both the clinical and preclinical phases of studies.
The development of the LC/MS interface has led to the development of new methods and techniques for bioanalysis. LC-MS Bioanalysis instrumentation includes the following systems: API 3000/4000/5000/6500, Tomtec Quadra 96-Well, and Hamilton StarLET. Bioanalysis projects are managed by experienced and skilled Principal Investigators. These teams are composed of Project Managers, Scientists, and chemists. The project teams assure your methods are perfectly validated, and that study samples are analyzed and reported in a timely and cost effective manner.

There are two types of approaches in LC-MS Bioanalysis, Top Down and Bottom Up. Top Down, also known as Intact Analysis, is almost new development in the bioanalysis but has gathered significance in recent years as of its ability to justify equivalent information to other approaches. In the Top down Approach, the biologics are directly applied to LC–MS analysis on a high-resolution mass spectrometer for the detection of the whole molecule. It is not a completely new concept and it has been used for the characterization of biologic products during manufacturing.
Bottom Up approach is a widely adopted and well-established workflow that has been commonly conducted in many bio labs. In a bottom-up approach, the biologics are digested by a proteolytic enzyme, typically trypsin, into peptides which are detected on a triple quadrupole (or high-resolution mass spectrometer) as surrogates of the protein.
The choice of assay typically depends on the sort of analytic, the desired assay sensitivity and most significantly the inquiries to be answered. If we have a tendency to square measure addressing a structurally stable biological, like an antibody, a bottom-up assay will offer the advantage of high sensitivity and superior property afforded by detection at amide level by LC–MS.
Liquid Chromotography
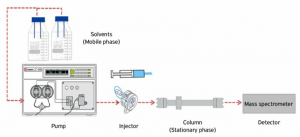
Liquid Chromotography is a methodology of physical separation in which the parts of a liquid mixture are distributed between 2 unmixable phases like Stationary and Mobile. There are five categories in practice of Liquid Chromotography,
- Partition Chromotography
- Adsorption Chromotography
- Ion-exchange Chromotography
- Size-exclusion Chromotography
- Affinity Chromotography
Reverse Phase (RP) mode of Partition chromatography technique is the most widely used variant in LC. A nonpolar (hydrophobic) stationary phase and a polar mobile phase are used in this process. In HPLC (High-performance liquid chromotography), 20 μl of the sample of interest is typically injected into the mobile phase stream, which is provided by a high-pressure pump. The analytes-containing mobile phase permeates the stationary phase bed in a certain direction. The components of the mixture are separated into mobile and stationary phases based on their chemical affinity.
Mass Spectrometry
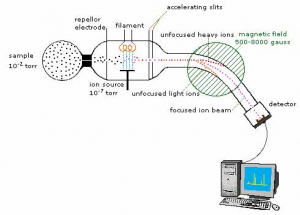
MS (mass spectrometry) is an approach for determining the mass-to-charge ratio (m/z) of charged particles (ions). These measurements are frequently used to determine the exact molecular weight of sample components. Although mass spectrometers come in a variety of shapes and sizes, they all use electric or magnetic fields to control the velocity of ions created by an analysis of interest and complete their m/z. Molecular weight determination, quantification of known chemicals, and resolution of structure and chemical characteristics of molecules are all common uses for mass spectrometers.
Do you want to know more about LC/MS for Regulated Bioanalysis? Join us at the Regulated LC/MS Bioanalysis Online MasterClass on 26th and 27th January, 2022.
Also check our In-house Training for developement of your employees and teams.
By Fazmi Zakam, Junior SEO Executive, GLC Europe, Colombo Office, Sri Lanka.
Get a feel for our events

Training Program for CMC Leaders - EU edition
14th September 2026 - 09th April 2027
Rich with practical insights and real-world applications
learn more >>
Training Program for CMC Leaders - US edition
14th September 2026 - 09th April 2027
Rich with practical insights and real-world applications
learn more >>
Advanced Stability Testing of Pharmaceuticals MasterClass - US edition
24-27 February, 2026
Increase the likelihood of studies receiving regulatory approval
learn more >>












