Advanced Pharmacovigilance MasterClass - EU edition
24-26 November, 2025
In depth assessment of all aspects of drug safety in ever changing regulatory environment
Through expert-led sessions, case studies, and interactive discussions, participants will gain valuable insights into the evolving landscape of drug safety and learn how to apply advanced methodologies in real-world settings.
24-26 November, 2025, Virtual
Time Zone: UTC/GMT+1 /CET
Overview
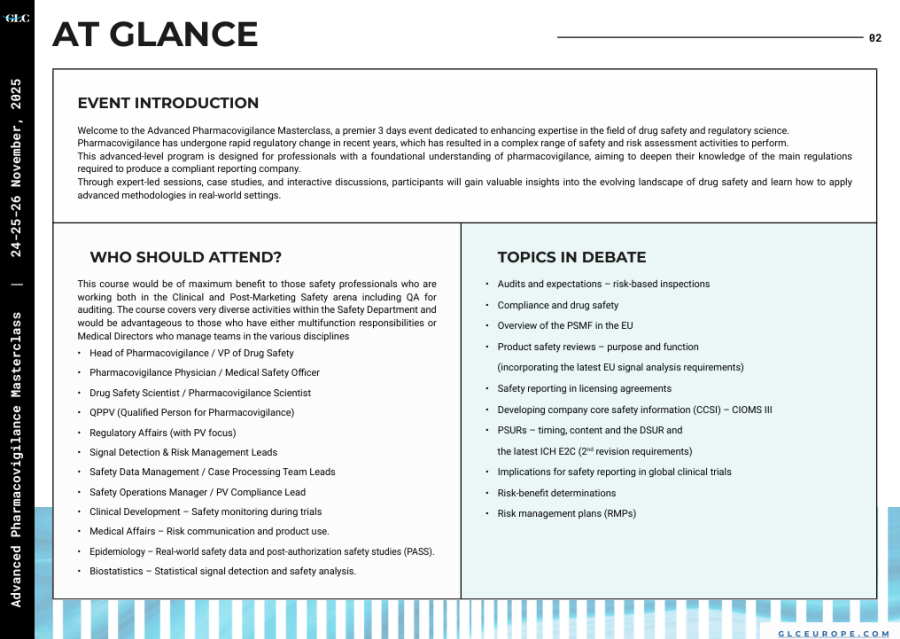
Welcome to the Advanced Pharmacovigilance Masterclass, a premier 3 days event dedicated to enhancing expertise in the field of drug safety and regulatory science. Pharmacovigilance has undergone rapid regulatory change in recent years, which has resulted in a complex range of safety and risk assessment activities to perform.
This advanced-level program is designed for professionals with a foundational understanding of pharmacovigilance, aiming to deepen their knowledge of the main regulations required to produce a compliant reporting company.
Through expert-led sessions, case studies, and interactive discussions, participants will gain valuable insights into the evolving landscape of drug safety and learn how to apply advanced methodologies in real-world settings.
#masterclass #glceurope #pharmaonlinemasterclass #globalleadingconferences #pharmacovigilance #signalmanagement #advancedpharmacovigilance #drugsafety #PSUR #RMP #PSMF #CIOMS #CCSI #ICHE2C
 WHO SHOULD ATTEND
WHO SHOULD ATTEND
Safety professionals who are working both in the Clinical and Post-Marketing Safety arena, including QA for auditing. The course covers very diverse activities within the Safety Department and would be advantageous to those who have either multifunction responsibilities or Medical Directors who manage teams in the various disciplines
-Head of Pharmacovigilance / VP of Drug Safety
-Pharmacovigilance Physician / Medical Safety Officer
-Drug Safety Scientist / Pharmacovigilance Scientist
-QPPV (Qualified Person for Pharmacovigilance)
-Regulatory Affairs (with PV focus)
-Signal Detection & Risk Management Leads
-Safety Data Management / Case Processing Team Leads
-Safety Operations Manager / PV Compliance Lead
-Clinical Development Safety monitoring during trials
-Medical Affairs Risk communication and product use.
-Epidemiology Real-world safety data and post-authorization safety studies (PASS).
-Biostatistics Statistical signal detection and safety analysis.
Testimonial
Our success stories
"High attention to detail in course content and very well delivered"
Simon Halsey
Product Development Manager
Essentra Packaging
United Kingdom
Our success stories
"Very good training led by two knowledgeable and open experts. Excellent insight given on many complex topics. Interactive and highly useful"
Aurelie Vivicorsi
USP PD Team Manager
Celonic AG
Switzerland
Our success stories
"Great course, impressed with the knowledge of the trainers and ability to answer wide variety of questions!"
Emilia Szwej
Manager, Senior Investigator
MT Sword Laboratories (BMS)
Germany
About GLC
Global Leadership Conferences began as an ambitious dream by three founders ten years ago. Today its an international series of interactive events, exploring the hottest topics in critical fields. Each year, thousands of professionals join us to challenge the status quo and learn innovative ways to create new solutions in Finance, Pharmaceutical, HR, Health & Safety, and Energy.
know more >14+
Years of experience
600+
Events organized
4,000+
Speakers
25,000+
Attendees

Other events you may like

Training Program for CMC Leaders - EU edition
27th October 2025 - 16th January 2026
Rich with practical insights and real-world applications
learn more >>
Pharma Contract Drafting MasterClass - EU edition
17-20 November, 2025
The principles of successful contract drafting
learn more >>



























